NEWS
A protein known as PERK has been
found to coordinate the communication
between the inside and the outside of the
cell. This finding opens up new avenues
for further research into treatments for
cancer, Alzheimer's, and diabetes.
Proteins such as insulin are properly
formed in the endoplasmic reticulum (ER),
one of the biggest membrane structures
in the cell. The ER works like an assembly
line and folds the proteins into a threedimensional shape that is essential for
them to function. When there is a problem
in the 'protein folding assembly line', the
accumulation of misfolded proteins can
lead to diseases such as Alzheimer's,
cancer, and diabetes.
An essential component of this proteinfolding factory is PERK. "This protein
is
known to play a crucial role in maintaining
ER functions and restoring them if
necessary," explains Patrizia Agostinis,
head of the KU Leuven Laboratory of Cell
Death Research & Therapy. "When PERK
detects protein folding errors in the ER it
prompts the nucleus of the cell to take
action."
Agostinis, Alex van Vliet, and other
team members have now discovered an
additional function of PERK. Agostinis
explains further, "We found that PERK also
coordinates the communication between
the protein folding factory (the ER) and the
skin of the cell (the plasma membrane).
When the protein-folding factory detects
low calcium levels, the plasma membrane
needs to let calcium flow back in. Calcium
is crucial for the proper functioning of the
protein folding factory - the ER, where
the calcium is stored - and for the overall
health of the cell. And this is where PERK
comes in: the protein establishes contact
between the two cell components so that
they can work together to restore the
calcium level."
Alex van Vliet adds, "This entire
process, which is regulated by PERK,
takes place in a matter of minutes or even
seconds. That's one of the reasons why
it went unnoticed until now. We used a
new method to reveal the underlying
mechanism, and were surprised to find
that PERK can control the movement of
the ER towards the plasma membrane by
modifying the skeleton of the cell."
The newly discovered role of
PERK opens up promising therapeutic
avenues. "But we must not get ahead of
ourselves," Agostinis emphasizes. "This
is fundamental research. Much more
work needs to be done before we can
even start thinking of new treatments that
target this new function of PERK."
UNEXPECTED PERK
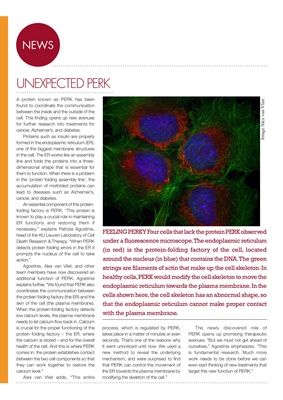
FEELING PERKY Four cells that lack the protein PERK observed
under a fluorescence microscope. The endoplasmic reticulum
(in red) is the protein-folding factory of the cell, located
around the nucleus (in blue) that contains the DNA. The green
strings are filaments of actin that make up the cell skeleton. In
healthy cells, PERK would modify the cell skeleton to move the
endoplasmic reticulum towards the plasma membrane. In the
cells shown here, the cell skeleton has an abnormal shape, so
that the endoplasmic reticulum cannot make proper contact
with the plasma membrane.
Image Alex van Vliet.