8
NEWS
Vertex trial resumes
Following a three month pause in early 2024, Vertex is
resuming the trial of VX-880, an investigational stem cell
therapy used to restore insulin production in people with
Type 1 diabetes.
Vertex paused the study in January 2024 after two
participants died. During the pause, an independent data
monitoring committee reviewed the data and determined
that the deaths were not related to the trial treatment,
and Vertex is now recruiting new participants again.
Brian Shelton, 66, one of the patients who died, was
said to be the first person 'cured' of Type 1 diabetes with
VX-880, having started to produce his own insulin in 2021,
six months after being treated with VX-880.
While the study was on hold, no new patients were
able to start VX-880 therapy, although, during the
pause, the existing participants continued their regularly
scheduled treatment with VX-880. Based on the latest
data analysis, all 14 current participants dosed with
VX-880 produced insulin. Previously, in October 2023,
Vertex shared that three participants had achieved insulin
independence, meaning their bodies were producing
enough insulin that they no longer needed insulin via
injection or pump
Though still in its early phases, study data supported
VX-880 as a promising and potentially revolutionary
treatment for Type 1 diabetes. Vertex has reported VX880 as being well-tolerated
with no serious side effects.
So far, the safety profile of VX-880 is similar to what
would be expected, given the use of immunosuppressants,
the surgical procedure to implant the cells, and the
participants' medical history.
The data has also shown promise for VX-24,
another Vertex stem cell therapy that does not require
immunosuppressants currently being tested in
clinical trials.
Transdermal drug delivery
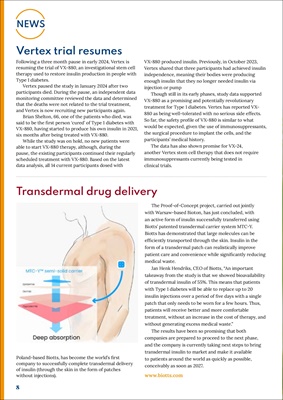
Poland-based Biotts, has become the world's first
company to successfully complete transdermal delivery
of insulin (through the skin in the form of patches
without injections).
The Proof-of-Concept project, carried out jointly
with Warsaw-based Bioton, has just concluded, with
an active form of insulin successfully transferred using
Biotts' patented transdermal carrier system MTC-Y.
Biotts has demonstrated that large molecules can be
efficiently transported through the skin. Insulin in the
form of a transdermal patch can realistically improve
patient care and convenience while significantly reducing
medical waste.
Jan Henk Hendriks, CEO of Biotts, "An important
takeaway from the study is that we showed bioavailability
of transdermal insulin of 55%. This means that patients
with Type 1 diabetes will be able to replace up to 20
insulin injections over a period of five days with a single
patch that only needs to be worn for a few hours. Thus,
patients will receive better and more comfortable
treatment, without an increase in the cost of therapy, and
without generating excess medical waste."
The results have been so promising that both
companies are prepared to proceed to the next phase,
and the company is currently taking next steps to bring
transdermal insulin to market and make it available
to patients around the world as quickly as possible,
conceivably as soon as 2027.
www.biotts.com